Which Ph Reading Is Suitable for Most Plants and Soil Microorganisms?
Introduction
Invasive Conflicting Plants (IAPs) take a neat impact on the structure and function of ecosystems. A growing trunk of literature has shown that diverse invasive plants decrease local found species variety, increase ecosystem productivity, change the rate of food cycling, affect human health and therefore touch on ecosystem services and human well-being (Richardson and van Wilgen, 2004; Pejchar and Mooney, 2009; Ehrenfeld, 2010; Vilá et al., 2011; Mannino and Balistreri, 2018). The characterization of IAPs and the study of the environmental factors underlying their success are therefore pivotal to develop effective IAP control measures.
Across climate, soil characteristics are believed to play an of import office in the survival and functioning of alien plants and therefore in successful invasion (Caplan and Yeakley, 2006). Soil reaction (pH), in particular, can exist considered a key variable due to its influence on many other soil proprieties and processes affecting constitute growth. Indeed, microorganism activity every bit well as nutrients solubility and availability are some of the about important processes that depend on pH. For instance, in acid soils, most micronutrients are more available to plants than in neutral-element of group i soils, generally favoring plant growth (Lončarić et al., 2008). However, some of these micronutrients, along with non-essential elements, can become toxic when their concentration is too large. In dissimilarity, in alkaline soils, although the availability of almost macronutrients is increased, phosphorus and micronutrient availability is by and large reduced and their lower levels tin adversely affect plant growth. Specifically, many constitute characteristics (i.eastward., traits) such as height, lateral spread, biomass, bloom size and number, pollen production, etc., are influenced past pH (Jiang et al., 2017).
IAPs usually possess broader tolerance to ecology weather, including pH (Dassonville et al., 2008; Hao et al., 2017), than crop and native plants, which have an optimum for pH mostly ranging from v.five to 6.5 (Islam et al., 1980; Köpp et al., 2011). This feature allows them to adapt to a great variety of soil types and thus to spread vigorously, as well colonizing environments not suitable for native species (Sǎrǎteanu et al., 2010). Despite the tolerance of some weeds to different pH having been reported, especially in agriculture, the affect of different soil pH on IAPs has been seldom studied so far (Gilbert and Lechowicz, 2005; Caplan and Yeakley, 2006; Zeng and Clark, 2013).
Among IAPs, Ambrosia artemisiifolia Fifty. (mutual ragweed) is a species of cracking concern in Europe. Since the nineteenth century, this species of North American origin has been accidentally introduced in Europe where information technology has naturalized and is at present considered an increasingly serious threat to both surround and man health (Montagnani et al., 2017). It is a fast-growing annual weed in ingather fields and a colonizer in open-disturbed areas, capable of producing considerable aboveground biomass at various pure stand densities (Patracchini et al., 2011; Fenesi and Botta-Dukát, 2012; Gentili et al., 2017). It also produces large corporeality of highly allergenic pollen, which represents one of the principal causes of pollinosis in many regions of the world (Smith et al., 2013). As for other IAPs, many factors contribute to the increasing spreading of the common ragweed. In particular, since it is a plant that mainly colonizes blank and disturbed soils, especially agriculture areas, abiotic factors related to the characteristics of soil tin can highly influence its distribution, particularly, soil pH, whose general importance for plant establishment, growth and maturation (i.e., reproductive potential and pollen production) has been largely acknowledged, but whose specific effects on A. artemisiifolia (and other species) have been poorly investigated to date. In controlled weather, Sang et al. (2011) demonstrated that A. artemisiifolia germination success exceeded 48% in solutions with pH values between 4 and 12, with maximum rates occurring in distilled h2o at pH five.57. In addition, in a field written report, Pinke et al. (2011) found that the highest mutual ragweed comprehend at the border of Hungarian sunflower fields occurred where the soil was acid (effectually pH5). In dissimilarity, mainly based on observations conducted in Austria, Essl et al. (2015) reported that A. artemisiifolia grows better in moderately alkaline metal weather, co-ordinate to Ellenberg indicator values. Similarly, Pignatti et al. (2005), indicated that in Italy the species has a wide ecological amplitude for soil pH measured with the Ellenberg and Landolt indicator values for soil reaction. Nonetheless, upwards to now, all these studies focused mainly on the distribution of the species in areas with different pH, and no data was available on the influence of soil pH on its growth and reproductive performances. Recently, some farmers (i.eastward., Bottega Agricola Fratelli Airoldi, Lombardy Region, Italia) who work on croplands highly invaded past A. artemisiifoila, observed that the addition of calcium hydroxide to the soil for improving its characteristics and increase its pH value, besides inhibits the growth of A. artemisiifolia. For all these reasons, although there may be other of import environmental factors affecting the fettle of A. artemisiifolia, nosotros focused our enquiry on soil reaction in lodge to understand its specific contribution. Hence information technology is possible to hypothesize that soil pH affects not only the distribution, just also the growth and the reproductive performance of this species, and can exist used to control its spread. In add-on, studies take demonstrated that also pollen allergenicity is strongly afflicted past environmental conditions (i.e., changes in temperature, relative humidity and calorie-free) during plant growth and flowering (Goto et al., 2004; Ghiani et al., 2016). Hence, information technology is possible to hypothesize that soil pH may likewise impact pollen allegenicity of A. artemisiifoila, but to the best of our knowledge, no investigation regarding the role of pH on this trait is currently bachelor in the literature.
In this study, we aimed to investigate how pH affects germination, growth-related traits, reproductive investment, pollen production and allergenicity of A. artemisiifolia. We grew plants in controlled conditions in a replicated experimental design and used prediction models to accurately estimate the species performance in relation to pH values (Mohebbi and Mahler, 1989; Robson, 1989; Kidd and Proctor, 2001; Hao et al., 2017). In particular, we used nonlinear models following a sigmoid pattern (i.e., logistic curves) to determine how soil pH affects the formation, growth rate of vegetative traits and reproductive investment of A. artemisiifolia (Yin et al., 2003; Sun and Frelich, 2011; Paine et al., 2012; Chen et al., 2014) and if soil pH has an effect on the pollen allergenicity of the species.
Materials and Methods
Institute Material and Preliminary Germination Test
All the experiments were conducted using A. artemisiifolia seeds collected in a ruderal surface area about the town of Brescia (northern Italy; North: 45°29′23″; E: 10°11′47″). Seeds were cold-stratified at 4°C for 3 months to overcome seed dormancy then planted in a tray containing autoclaved natural soil.
Preliminary germination tests were performed at unlike pH values in 1% found agar: pH3, pH4, pH5, pH6, pH7, pH8, and pH9 were considered. The pH values were adjusted with 1 mol/50 HCl (low values) and NaOH (high values) according to Sang et al. (2011). Before the test, seeds were sterilized in a solution of distilled water added with sodium hypochlorite (NaClO) at 3% for 2 min; seeds were then washed and dried. For each pH, iii Petri dishes containing 30 seeds were set up. To prevent contamination and h2o loss, Petri dishes were sealed with Parafilm. The 3 replicates of the germination plates for each pH were put in growth chambers (model Sanyo MLR-350; Sanyo Electric Co., Japan), in controlled condition of light (12 h dark/12 h low-cal, 150 μmol m−2 s−one) for thirty days at a temperature of 25°C. Petri dishes were checked under a binocular microscope for germination weekly and seeds were recorded as germinated once the radicle protrusion occurred. At the end of the germination test, a cutting test was carried out on non-germinated seeds to assess the presence of the embryo. Calculations of the concluding germination ratio did non include non-germinated empty seeds.
Soil Training and Establish Growth
Based on the effect of different soil pH on the growth of vegetative traits, reproductive investment and pollen allergenicity of A. artemisiifolia was tested in pots in growth chambers nether controlled conditions of lite (12 h dark/12 h light, 150 μmol m−2s−1; humidity: 65%) at 25°C for 3 months and one-half (104 growing days), from 9th April to 22th July, 2015. A randomized complete block pattern experiment with 5 replicates (number of individuals was limited by the limited space within the growth chamber) was carried out to examine the effect of soil pH on institute growth.
To reproduce optimal abiotic ecology conditions for the species germination and growth, plantlets germinated in natural soil were transferred into plastic pots (2,000 ml capacity) filled with the same natural soil subsequently arranged at unlike pH values. Specially, we used a soil stock collected from an agriculture expanse highly invaded past ragweed, at Busto Arsizio (Varese, Italy; N: 45°35′59″; E: 8°52′29″), in February 2015. A soil sub-sample was subjected to a physico-chemical characterization at the soil laboratory of the Milan-Bicocca University (see Supplementary Material S1).
We prepared three dissimilar soils for plant growth at the optimal pH values selected subsequently the preliminary germination tests on agar: pH5 (acid), pH6 (sub-acid), and pH7 (neutral). To obtain the selected pH values from the natural soil, a liming method was used co-ordinate to literature (Dark-brown et al., 2008; Thompson et al., 2016). Particularly, calcium hydroxide solutions [Ca(OH)2] was added to the natural soil stock, with a pH value of v.0, to get two amended soils at pH6 and pH7 (meet Supplementary Fabric S2).
During the whole growth period of plants, the pH value of the prepared soils was measured and monitored weekly (see Supplementary Cloth S3). In gild to avoid disturbance to the growing establish, we collected 10 one thousand of soil in the well-nigh lateral function of the pot with a small spoon, after moving the superficial part the soil (about 1 cm). The pH of the soil was potentiometrically measured in the supernatant suspension of a one:2.5 soil:liquid mixture using the pH-meter, model Eutech pH 700 (Eutech instruments); both distilled h2o and neutral salt (KCl) solution were used: (a) x.0 g of fine earth (>two mm) were added with 25 ml of demineralized water or one K solution of KCl in a 50 ml chalice; (b) the soil/water intermission was shacked with a glass rod and leave to remainder and decant for at least 2 h; (c) the pH electrode was immersed in the articulate part of the suspension and the pH value read after stabilization of the measurement.
Vegetative and Reproductive Traits
We nerveless weekly data on vegetative and reproductive plant traits on the five plants grown in each growth chambers:
(a) plant meridian (cm), measured from the plant crown to the maximum growing point of the chief co-operative;
(b) lateral spread (cm), measured every bit the maximum diameter of the found;
(c) maximum leaf length (cm), measured from the petiole base to the foliage noon;
(d) maximum leaf width (cm);
(eastward) presence or absenteeism of floral buds;
(f) number of male racemes (the spikes with male flower heads);
(g) male person raceme length (cm; measured at the terminate of anthesis);
(h) pollen release, monitored as presence or absence during time;
(i) dry out weight of aerial biomass (yard), measured at the cease of the growth period.
Pollen Collection and Allergenicity
Mature pollen of each found was recovered in transparent collectors, past covering three male person inflorescences (when present) with a modified ARACON system (Lommen et al., 2017) until 10 weeks afterward the outset of the treatments.
Slot blot technique was applied to assess the whole allergenicity of pollen nerveless from the different racemes, using commercial certified pollen of A. artemisiifolia (Allergon®) every bit standard. Soluble protein extracts were prepared according to Aina et al. (2010). Equal volumes of these extracts, containing an identical amount of proteins, were spring to nitrocellulose membrane and starting time stained with Ponceau S staining solution [0.1% (westward/v) Ponceau S in 5% (v/v) acetic acid] to assess the corporeality of proteins loaded in each well. Membranes were so used to evaluate the immunoreactivity of the different pollen extracts to a puddle of sera from ragweed allergic patients, previously selected (Asero et al., 2014). Paradigm analysis was applied to quantify reactivity signals. The integrated optical density (IOD) of immunoreactive spots with respect to the IOD of standard (Allergon®) was measured. At least three different samples for each racemes were analyzed.
Statistical Methods
The final ratio of germinated seeds between each pH was compared using assay of variance (ANOVA) test, followed by post-hoc Tukey multiple comparing test.
We used the FlexParamCurve v. 1.five–3 (Oswald et al., 2012) bundle in R 3.3.2 (R Cadre Squad, 2016) for modeling growth trajectories of common ragweed. This parcel provides tools that facilitate plumbing equipment parametric curves in nonlinear modes, which is computationally efficient and allows the interpretation of parameters of biological significance even on relatively small datasets (Oswald et al., 2012). Despite the package is designed to fit a large family of growth curves, including not-monotonic ones, we parameterized the model for fitting the four-parameter generalized logistic bend (merely "logistic curves" hereafter) past setting modno = 12 in the SSposnegRichards procedure.
In detail, the four-parameter generalized logistic curve is described by the equation
Where y is the estimated value at time t, A is the asymptote of the growth trajectory, 1000 is the rate at which the slope of the bend changes with time, i is the inflection indicate, respective to the time at which the growth is fastest, and m is the shape parameter of the generalized logistic bend.
We used the nlme process in the nlme parcel (Bates, 2005) to fit nonlinear mixed modes (NLMMs) for investigating whether values of the A, k, i, and m parameters of the curve were affected by soil pH. NLMMs allow for a big flexibility in the parameterization of both the fixed and the random part of the model, simply this flexibility likewise makes information technology difficult to appraise the optimal construction of the model. Following a similar procedure described in Sicurella et al. (2014) and Morganti et al. (2017), nosotros therefore ran preliminary analyses to assess which parameter(south) showed variability according to soil pH (entered every bit a 3-level factor), and which parameter(s) showed large amidst-individual variability, and should therefore exist included as random effect(due south). These preliminary analyses were run by starting time interpolating logistic curves to data of each institute separately and noting the estimated value of individual parameters. Then, nosotros used ANOVA models, corrected for inhomogeneity of variance whenever necessary, to exam for variation in each parameter according to pH. When these analyses revealed significant variation, the result of pH was maintained in the final NLMM, while it was excluded otherwise. For instance, ANOVA models showed that parameters A, k and i from individual plant height model significantly varied among pH levels, while k parameter did not. Thus, the NLMM allowed A, chiliad and i to vary with pH, but not m. We also plotted the range of parameters from curves fitted to individual plants and, in the NLMMs, immune for random variation in those parameters which, at a visual inspection, showed large heterogeneity (see as well Sicurella et al., 2014; Morganti et al., 2017). Finally, we controlled for heteroscedasticity past assuming a variation of the variance with time according to an exponential office, as suggested in Oswald et al. (2012) in all models except for that of leaf width because a model assuming homoscedasticity had a lower AICc value, indicating a better fit (details not shown).
Differences in found dry weight, number of inflorescences, inflorescence size (including only plants that did produce inflorescences), and time to pollen emission between plants grown at unlike pH were tested in ANOVA models, generalized linear models assuming a Poisson distribution and corrected for overdispersion, linear mixed models and parametric survival regression models assuming a negative exponential distribution (Kleinbaum and Klein, 2005). Institute dry weight was entered every bit a covariate in all the other models. Plant identity was included as a random grouping factor in the mixed model of inflorescence size to business relationship for repeated measures taken on the aforementioned plant. This model was besides corrected for heteroscedasticity by bold that variance increased exponentially with plant dry out weight. Time to pollen emission was considered an interval censored variable (Kleinbaum and Klein, 2005) equally pollen production was evaluated merely during periodic visits. In detail, no plant emitted pollen before 12/6, while some did not produce pollen earlier the end of the experiment (15/7). Intervals where therefore considered right censored, but non left censored (Kleinbaum and Klein, 2005).
Analyses where run in R 3.3.two (R Cadre Team, 2016) with the "nlme" (Pinheiro and Bates, 1995), "survreg" (Therneau and Grambsch, 2000), and "multcomp" (Hothorn et al., 2008) libraries.
Results
Germination Test
Under the faux conditions significant differences in the germination percentage were observed betwixt the tested pH values. Every bit a general dominion, pH5 (acrid), pH6 (sub-acid), and pH7 (neutral) performed meliorate and exhibited germination ratios in a higher place (or effectually) 50% (Effigy 1). These values of pH were retained for further analyses regarding the growth rate of institute traits and allergenicity.
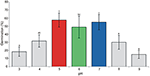
Figure one. Germination percentage (means + st. dev.) of A. artemisiifolia under different pH values (ANOVA: F = 11.69; df = half-dozen,26; P < 0.001). Different letters indicate pregnant differences of germination at P < 0.05 level (Tukey multiple comparison test).
Growth Rate and Constitute Traits
We collected eleven measures for fifteen plants. One plant, grown at pH7, lost leaves before the concluding mensurate, thus we could not measure lateral spread, leaf length and width, but we measured summit because the establish was notwithstanding alive. Sample size is therefore 165 measures for plant peak and 164 measures for the other parameters.
Concluding NLMM of common ragweed peak indicated that at pH7 plants were shorter than those grown at pH5 and pH6 as suggested by the significant difference in A parameters of the generalized logistic curves (Tabular array 1). In improver, plants grew faster at pH5 than at pH6 and pH7 as indicated past the difference in k parameters of the growth curves. Overall, growth trajectory of plants grown at pH5 and pH6 were similar, while that of plants grown at pH7 differed (Effigy 2A).
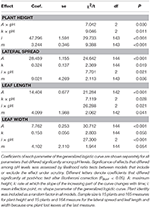
Tabular array ane. Final NLMM of the growth trajectories.
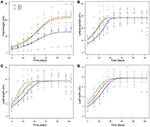
Figure 2. Generalized logistic growth curves of vegetative traits [plant meridian (A), lateral spread (B), leaf length (C) and leafage width (D)] of A. artemisiifolia according to different pH values (pH5, pH6 and pH7, displayed in different colors) at 25°C.
Common ragweed plants grown at unlike pH showed similar final lateral spread and final size of leaves, as suggested past the fact that A parameter did not differ among pH levels in all models. Notwithstanding, foliage development was slower at pH7, as indicated by the fact that i parameter, indicating when curves reach the inflection signal, was larger for plants grown at pH7 than at lower pH in all models (Tabular array ane, Figures 2B–D). At pH6, however, establish leaves seemed to grow more quickly than at pH5, as indicated by a significantly lower value of i parameters of models of leaf length and width (Tabular array 1). In dissimilarity, no significant divergence was observed in i values of the model of lateral spread (Tabular array ane). Leaves seem as well to increase in size at different rates at different pH, as suggested by the significant interaction betwixt k parameter and pH. However, post-hoc tests could not identify any significant pairwise difference in these parameters after Bonferroni correction (Table ane).
Overall, growth curves showed that mutual ragweed canopies grew at similar rate at pH5 and pH6. (Table 1, Figure 1B), but more slowly at pH7. Leaves seem to grow fast at pH6, at intermediate rate at pH5 and slow at pH7 (Figures 2C,D).
Plant dry weight did not differ significantly between plants grown at different pH [F (2,12) = 1.213, P = 0.331; Figure iii].
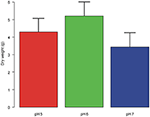
Figure iii. Dry weight of the aerial biomass (ways + st. dev.) of A. artemisiifolia under different pH values. No significant differences were detected amongst the treatments.
Reproductive Investment
The number of inflorescences increased with constitute dry weight (coef = 0.752 ± 0.140 SE, t = v.391, df = 11, P < 0.001; Effigy four) and differed significantly betwixt plants grown at different pH (F = two.848, df = two,11, P = 0.031; Figure four). This departure was due to the fact that plants grown at pH7 never produced inflorescences, while no difference in the number of inflorescences was found between plants grown at pH5 and pH6 (F = 0.003, df = one,7, P = 0.958).
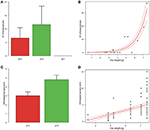
Effigy four. Means number of inflorescences and inflorescence size of A. artemisiifolia under unlike pH values (A–C). At pH7 the plants did not produce any inflorescences. Relationships between number of inflorescences and inflorescence size with dry weight of the aerial biomass (B–D); both parameters increased significantly with dry weight (P < 0.001; P = 0.047, respectively).
Inflorescence size increased with plant dry weight (coef = 1.168 ± 0.358 SE, t = three.266, df = 11, P = 0.047; Figure four), only did not differ amongst plants grown at pH5 and half dozen (t = 0.215, df = 85, P = 0.843; Figure four).
Time to pollen emission decreased with plant dry weight (coef = −0.893 ± 0.330 SE, z = −2.703, P = 0.006), merely did not differ between plants grown at different pH (log-likelihood ratio examination: χii = 5.379, df = ii, P = 0.068).
Pollen Total Allergenicity
Pollen from plants grown at pH five and 6 was assessed by slot absorb technique in lodge to preserve protein conformation, on which IgE binding may depend. Identical corporeality of proteins from pollen extracts were bound on a nitrocellulose membrane and subjected to immunoreaction with a sera mix from selected ragweed allergic patients. The Figure 5A shows a representative membrane afterwards immunodetection. Prototype analysis was applied to quantify immunochemical signals: the integrated optical density (IOD) of immunoreactive sposts with respect to the IOD of standard (sample IOD/standard IOD) was measured. At least three protein extracts from each plant were analyzed and the mean results of five independent experiments were calculated and statistically elaborated (Figure 5B).
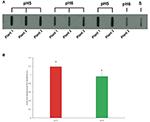
Figure v. (A) Representative slot absorb membrane probed with a pool of selected patient sera showing the full allergenicity of pollen samples collected from plants grown in soils at pH5 and pH6. Pollen proteins obtained from single plants by contained extractions were loaded. (S) Standard (protein extract from commercial pollen, Allergon); (B) Hateful total allergenicity of pollen collected from plants grown at pH5 and pH6. Paradigm analysis was applied to measure the integrated optical density (IOD) of immunoreactive spots with respect to the IOD of the standard (sample IOD/standard IOD). Different letters indicate significant differences between treatments at P < 0.05 level.
On average, all the pollen samples collected from plants grown at pH5 showed a statistically higher IgE-bounden signal, ranging from i.12 to 1.25, than pollen from plants grown at pH6, whose immunochemical signal ranged from 0.86 to 1.03 (P < 0.05).
Discussion
This study demonstrates for the first time that growth and reproductive performances of the IAP A. artemisiifolia were greatly affected past soil pH in controlled conditions. Specifically, results ostend that A. artemisiifolia is able to germinate and abound in soils with different pH, just its success, in terms of growth of vegetative traits and reproductive investment, increases when the soil reaction is slightly acid, at pH6. On the contrary, the total pollen allergenicity was lower at pH6 than pH5, the only two pH values at which plants produced flowers then pollen. Since, there is a number of important environmental factors that may control the distribution of common ragweed (i.e., nutrients, temperature, moisture, etc.) and our work only focused on the factor soil pH in controlled conditions, we recognize it may have some limits. Despite this, nosotros would bespeak out that: (a) soil pH is known to control the uptake of macro- and micronutrients (Due north, Mg, and then on) from soil so information technology is a quite of import factor to be monitored, especially for invasive plants; (b) we used natural soil for growing plants and measuring the pH; this selection made experimental weather condition more than shut to those of field atmospheric condition and so the subsequent results useful for futurity field experiments regarding the species' control; (c) we observed in a crop field (as specified in the introduction) that the amendment of soil using calcium hydroxide highly reduced the species growth.
The method we used in our test to modify the original reaction status of soil, which implies the addition of calcium hydroxide to increase pH value, tin can have changed the original quantity of calcium. Thus, calcium could act as confounding factor in agreement the effect of pH on plants. Although calcium is not a major nutrient, information technology plays a fundamental office in many physiological processes such as the stabilization of cell wall structures, the function of a major secondary-messenger molecule in plants under different developmental cues, the participation in mechanisms of water and food uptake, etc. (White and Broadley, 2003). However, from a static signal of view, the soil pH is more often than not more dependent on elements involved in exchange circuitous (H+ and cations) which buffers possible variations of pH through exchanges betwixt soil and soil solution (Pansu and Gautheyrou, 2003). As a effect, the addition of calcium hydroxide were intended to reproduce conditions like to field ones in which calcium is generally the most representative cation in soil substitution complex and to simulate the actual arrangement regulating pH values.
Germination, Growth of Vegetative Traits, and Reproductive Investment
The germination rate of A. artemisiifolia was higher at pH5 to pH7 than at lower or college pH values. These results are in accordance with those of Sang et al. (2011) who establish the optimum pH of germination between pH5 and pH8. In general, among the intermediate values of pH tested in this study for the subsequent analyses (growth bend and reproductive investment), plants grown at pH5 and pH6 performed improve than those grown at pH7.
With regard to vegetative traits, the shortest height as well equally the slowest growth charge per unit for all vegetative traits were recorded at pH7. This results are in disagreement with those of an old work of Turner (1928) reporting that A. artemisiifolia was more abundant and taller at neutral–slightly alkaline soil (pH 7.0–7.iii) than plants grown in sub-acid and acid soils (below pH7). Tessmer et al. (2013) institute that slower growth rate correlated with a reduced photosynthetic efficiency in different populations of Arabidopsis thaliana. Nevertheless, in our results although A. artemisiifolia grown at pH7 exhibited a slower growth for all traits than at pH5 and pH6, the final values of traits related to leafage size (length, width and lateral spread) did not significantly differ among pH values, indicating a swell ability of the plant to grow (i.east., adapt), at a slower charge per unit, at less suitable pH conditions (pH7 in our case). Physiological mechanisms of adaptation of plants to non-optimal soil pH are well-known in literature. Particularly, root-induced changes in the rhizosphere occur through the release of charges carried by H+ or OH− to balance cation–anion uptake at the soil–root interface (Hinsinger et al., 2003). This behavior is consistent with observations we made in our study, since the pH of soil conspicuously decreased over time at all the pH values monitored (data not shown). However, observing the whole dataset of vegetative found traits, plants grown at pH7 exhibited the everyman absolute value of biomass (fifty-fifty if not significantly unlike from pH5 and pH6), in addition to the lowest values of plant height and velocity of growth in add-on to the lack of male inflorescences. Likely a trend, not captured by our information, indicating less vigor of the species at pH7 can be invoked and should be taken into business relationship.
In whatever case, these results should be advisedly evaluated considering some confounding factors relating to the soil ecosystem: (a) soil pH is known to influence the availability and uptake of a micronutrient like Mg that is implicated in the institute'southward photosynthetic efficiency (Dighton and Krumins, 2014). For example, at high pH, Ca, and Mg tend to form less or not bachelor compounds when reacting with P and many micronutrients (Hairdresser, 1995); (b) complex interactions between biotic (i.e., bacteria and fungi) and abiotic factors that occur inside the soil ecosystem.
Key elements of soil useful for plants growth are nitrogen (Northward), potassium (K) and phosphorous (P) that unlike plants species tin preferentially blot co-ordinate to pH. For example, equally regards N content, plants can adsorb it in the forms of ammonium (NH) or nitrate (NO), according to soil pH (Serna et al., 1992; Abbasi et al., 2017). Since A. artemisiifolia is a considered a nitrophilic plant (Qin et al., 2014; Skálová et al., 2015) nitrogen very likely played a major function in influencing the institute growth in our experiment, also because that the natural soil we used for plants exhibited good concentrations of nitrogen (meet Supplementary Material S1). Specially, our results support the findings of Nádasi and Kazinczi (2011) that A. artemisiifolia grows better in sub-acrid soils (pH = 5.87) with higher ammonium-nitrate content than in neutral–slightly alkaline metal soils (pH = seven.26) with lower ammonium-nitrate content. Nonetheless, Leskovsek et al. (2012) observed that pure stands of A. artemisiifolia plants grown in field (at pH 6.half dozen) and greenhouse experiments (in peat moss; pH not indicated), produced considerable biomass and seeds nether various densities and nitrogen rate.
Contrary to our ascertainment, in another experiment involving other widespread/ruderal species (i.e., Alliaria petiolata and Sonchus arvensis) plants were capable of performing better, in terms of biomass, in less acid soils, toward pH7 (Zollinger and Kells, 1991; Anderson and Kelley, 1995) confirming a species-specific beliefs of plants for nutrient assimilation with respect to soil reaction.
With regards to reproductive investments, at pH6 and pH5 A. artemisiifolia plants showed similar trends too in terms of number of inflorescences and inflorescence size while at pH7 they did non produce any inflorescence, confirming the tendency observed for vegetative traits, i.e., at pH7 plant exhibited a worst performance. This beliefs of the plants at pH7 could too be due to the issue of an backlog of calcium hydroxide after the manipulation of the natural soil we used in our experiment. With regards to fourth dimension to pollen emission it significantly decreased with establish dry weight (shorter at pH5 than pH6), as expected.
The influence of pH on the reproductive investment has been already observed for other species (no literature information were found for A. artemisiifolia). For instance, in a work on the effect of unlike pH values (from 4.5 to viii) on the vegetative and reproductive growth of Rose cv., the best plant performance, in terms of number of buds, was obtained at pH6.5 and the lowest one at pH8 (Rosta and Rezaei, 2014). In dissimilarity, Lankinen (2000) found that depression pH (pH4) had a negative upshot on production of Viola tricolor flowers and seeds, which decreased of about 18 and 33% with respect to intermediate pH values.
Our results highlight that intermediate/slightly acid values of pH are in general most suitable for the growth and reproduction of A. artemisiifolia. Most constitute nutrients are known to be optimally bachelor to plants at intermediate/sub-acid pH ranges and are uniform to plant root growth (Jensen, 2010). Plants growing in likewise acrid or as well calcareous (i.e., alkaline metal) soils greatly change their uptake power of micro- and macronutrients and are constantly exposed to either mineral deficiency or metal toxicity (Ramírez-Rodríguez et al., 2005). Consequently, a non-optimal soil pH condition for a plant can affect its growth and reproductive performances, equally we accept observed in this study for A. artemisiifolia grown at pH7. In whatsoever example, also reproductive investment in response to different pH ranges is species-specific equally a result of evolutionary history and adaptation ability to environment of each species (Ware, 1990; Zeng and Clark, 2013; Offord et al., 2014).
Pollen Allergenicity
In this work, the soil pH at which a plant was grown affected common ragweed pollen allergenicity, which, in our experimental condition, was lowest at pH6. Unfortunately, no specific studies on the effects of soil pH on pollen allergenicity were performed to date. However, previous studies demonstrated that environmental variability and biotic/abiotic stress led to differences in the amount and type of pollen allergens. For instance, Ghiani et al. (2012) reported an increased allergenicity of pollen of A. artemisiifolia populations exposed to road traffic pollution. Climate change was indicated to impact pollen allergenicity determined by a higher concentration of the Amb a 1 allergen in pollen of plants exposed to higher temperatures and drought (El Kelish et al., 2014; Ghiani et al., 2016). Cloutier-Hurteau et al. (2014) measured the transfer of trace elements (i.e., Ba, Cd, Cr, Cu, Mn, etc.) from soil to pollen of A. artemisiifolia plants growing in ruderal sites in society to validate the affect of these elements on homo wellness, as possible explanation for the increase of allergy symptoms within industrialized areas. They establish positive relationships betwixt the concentration of some trace elements (Cd Ni and Atomic number 82) in pollens and in soil or roots. Unfortunately, they did not measure the allergenicity of those pollen grains; moreover they did non find any relation of the trace elements concentration in pollen grain with soil pH probably due to the limited pH range of the investigated soils (vii.31–8.39 range) likewise as to the loftier pH values that are unfavorable to chemical element mobility in soils. In our experiment, we can suppose that the addition of calcium hydroxide to soil in order to increase pH from 5 to six interfered with pollen allergenicity. Indeed, nosotros noticed a higher amount of flavonoids in pollen extracts from plant grown at pH6 probably produced to face the presence of calcium. This higher amount of secondary metabolites likely afflicted the IgE bounden explaining the lower allergenicity detected for pH 6 pollen.
Implication for A. artemisiifolia Direction
Despite the fact that our results in controlled conditions signal better performances of A. artemisiifolia in sub-acid soil conditions, the observations concerning the distribution or abundance of the species are inconsistent in field studies. Several authors found the highest abundance of common ragweed in acrid soil, toward pH5 (Ujvárosi, 1973; Szigetvári and Benkö, 2008; Pinke et al., 2011; Li et al., 2014). In contrast, other authors institute that the presence of A. artemisiifolia is meliorate related to neutral moderately alkaline status, toward pH7 and pH8 (Turner, 1928; Fumanal et al., 2008; Essl et al., 2015). However, it should be noted that in such studies the vegetative vigor and the reproductive performances of the found in the growth sites were non reported. The inconsistency of field studies on pH preference of weeds has been related to the covariation of pH range with climatic gradients (annual rainfall and temperature; Pinke et al., 2011, 2012). In our study, a neutral soil, obtained after the improver of calcium hydroxide to a natural acid soil, inhibited the emission of flowers (besides the plant top) during the ascertainment period. This result supports field observations by Italian farmers working on croplands highly invaded by A. artemisiifolia regarding the inhibiting upshot of the improver of calcium hydroxide on the growth of the species.
In the management of IAPs, manipulating the soil attributes is one of the strategies to accomplish a successful control, especially in agricultural environments. Particularly, nutrient and soil nitrogen management, highly dependent on soil pH values, or the addition of activate carbon take been used to achieve desired soil properties, and thus plant communities resistant to invasion (Kulmatiski and Bristles, 2006; Vasquez et al., 2008). Although we admit the limitations of simply testing the effects of soil pH in controlled conditions, our study suggests that further in-field research on the effects of liming on the growth and performances of A. artemisiifolia and other IAPs should be carried out, in society to examination effective control measures in agricultural environments. Species-specific approaches, may be implemented by applying soil liming methods (that may accept management problems and loftier costs) also tested in combination with other restoration methods (such equally N management, plowing, herbicide awarding, etc.) to enhance resistance of soils and native plant communities to numerous IAPs. In fact, it is surprising that the effect of invasive plants on soil pH has been investigated in numerous circumstances (Ehrenfeld, 2003), but not the opposite.
Interesting findings of our experimental study are that: (a) in not optimal pH conditions (pH7 in our study) A. artemisiifolia does not produce buds and inflorescences; in sub-optimal pH atmospheric condition for growth (pH5) the length of the pollen emission is reduced compared with the optimal pH weather condition (i.eastward., pH6), even if an reverse pattern was observed for pollen allergenicity. These factors should exist considered and may have possible implications during the evaluation of wellness take a chance linked to pollinosis.
Author Contributions
RG and SaCi conceived and designed the experiments. CM and SaCa conducted laboratory analyses. RG and RA analyzed the data and wrote results. RG and SaCi wrote the manuscript (Introduction and Discussion); all authors provided editorial advice and revised manuscript.
Funding
This study was funded past Fondazione Banca del Monte di Lombardia (Projection: Invasione biologica delle specie allergeniche del genere Ambrosia Fifty. in Lombardia: distribuzione dettagliata, pericolosità due east metodologie finalizzata contrastarne la diffusione) and past LIFE fiscal instrument of the European Committee to Lombardy Region LIFE14 IPE IT 018GESTIRE2020-Nature Integrated Direction to 2020.
Conflict of Involvement Statement
The authors declare that the enquiry was conducted in the absence of any commercial or financial relationships that could be construed as a potential disharmonize of interest.
Acknowledgments
We would like to thank Enrico Casati, Silvia Ciappetta, Alessandra Ghiani, Fabio Moia, and Valentina Rodio, for their technical support during laboratory analysis.
Supplementary Material
The Supplementary Fabric for this commodity can be constitute online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.01335/full#supplementary-textile
References
Abbasi, H.Northward., Vasileva, V., and Lu, X. (2017). the influence of the ratio of nitrate to ammonium nitrogen on nitrogen removal in the economical growth of vegetation in hybrid constructed wetlands. Environments iv:24. doi: 10.3390/environments4010024
CrossRef Full Text | Google Scholar
Aina, R., Asero, R., Ghiani, A., Marconi, M., Albertini, East., and Citterio, Southward. (2010). Exposure to cadmium-contaminated soils increases allergenicity of Poa annua Fifty. Pollen. Allergy 65, 1313–1321. doi: ten.1111/j.1398-9995.2010.02364.10
PubMed Abstract | CrossRef Full Text | Google Scholar
Anderson, R. C., and Kelley, T. M. (1995). Growth of garlic mustard (Alliaria petiolata) in native soils of dissimilar acidity. Trans. Ill. Land Acad. Sci. 88, 91–96
Google Scholar
Asero, R., Bellotto, E., Ghiani, A., Aina, R., Villalta, D., and Citterio, South. (2014). Concomitant sensitization to ragweed and mugwort pollen: who is who in clinical allergy? Ann. Allergy Asthma Immunol. 113, 307–313. doi: 10.1016/j.anai.2014.06.009
PubMed Abstract | CrossRef Full Text | Google Scholar
Barber, Southward. A. (1995). Soil Food Bioavailability: A Mechanistic Approach. New York, NY: John Wiley & sons.
Google Scholar
Bates, D. (2005). Plumbing fixtures linear mixed models in R. R News 5, 27–xxx.
Google Scholar
Brown, T. T., Koenig, R. T., Huggins, D. R., Harsh, J. B., and Rossi, R. Due east. (2008). Lime furnishings on soil acidity, crop yield, and aluminum chemical science in direct-seeded cropping systems. Soil Sci. Soc. Am. J. 72, 634–640. doi: 10.2136/sssaj2007.0061
CrossRef Full Text | Google Scholar
Caplan, J. South., and Yeakley, J. A. (2006). Rubus armeniacus (Himalayan blackberry) occurrence and growth in relation to soil and light conditions in western Oregon. Northwest Sci. 80, 9–17.
Google Scholar
Chen, D., Neumann, M., Friedel, Due south., Kilian, B., Chen, M., Altmann, T., et al. (2014). Dissecting the phenotypic components of crop plant growth and drought responses based on high-throughput image assay. Establish Cell. 26, 4636–4655. doi: 10.1105/tpc.114.129601
PubMed Abstract | CrossRef Full Text | Google Scholar
Cloutier-Hurteau, B., Gauthier, S., Turmel, Chiliad.-C., Comtois, P., and Courchesne, F. (2014). Trace elements in the pollen of Ambrosia artemisiifolia: what is the effect of soil concentrations? Chemosphere 95, 541–549. doi: 10.1016/j.chemosphere.2013.09.113
PubMed Abstruse | CrossRef Full Text | Google Scholar
Dassonville, N., Vanderhoeven, South., Vanparys, V., Hayez, M., Gruber, Due west., and Meerts, P. (2008). Impacts of alien invasive plants on soil nutrients are correlated with initial site atmospheric condition in NW Europe. Oecologia 157, 131–140. doi: ten.1007/s00442-008-1054-6
PubMed Abstract | CrossRef Full Text | Google Scholar
Dighton, J., and Krumins, J. A. (2014). Interactions in Soil: Promoting Plant Growth. New York, NY: Spinger.
Google Scholar
Ehrenfeld, J. Grand. (2003). Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems vi, 503–523. doi: 10.1007/s10021-002-0151-3
CrossRef Full Text | Google Scholar
Ehrenfeld, J. Thousand. (2010). Ecosystem consequences of biological invasions. Annu. Rev. Ecol. Evol. Syst. 41, 59–80. doi: x.1146/annurev-ecolsys-102209-144650
CrossRef Full Text | Google Scholar
El Kelish, A., Zhao, F., Heller, Due west., Durner, J., Winkler, J. B., Behrendt, H., et al. (2014). Ragweed (Ambrosia artemisiifolia) pollen allergenicity: superSAGE transcriptomic analysis upon elevated COtwo and drought stress. BMC Constitute Biol. xiv:176. doi: 10.1186/1471-2229-14-176
PubMed Abstract | CrossRef Full Text | Google Scholar
Essl, F., Bir,ó, K., Brandes, D., Broennimann, O., Bullock, J. Yard., Chapman, D. S., et al. (2015). Biological flora of the british isles: Ambrosia artemisiifolia. J. Ecol. 103, 1069–1098. doi: 10.1111/1365-2745.12424
CrossRef Full Text | Google Scholar
Fenesi, A., and Botta-Dukát, Z. (2012). Phenotypic divergences induced by dissimilar residence time in invasive common ragweeds. J. Plant Ecol. 5, 174–181. doi: 10.1093/jpe/rtr022
CrossRef Full Text | Google Scholar
Fumanal, B., Girod, C., Fried, Yard., Bretagnolle, F., and Chauvel, B. (2008). Can the big ecological aamplitude of Ambrosia artemisiifolia explain its invasive success in French republic? Weed. Res. 48, 349–359. doi: 10.1111/j.1365-3180.2008.00627.ten
CrossRef Full Text | Google Scholar
Gentili, R., Montagnani, C., Gilardelli, F., Guarino, G. F., and Citterio, S. (2017). Let native species take their course: Ambrosia artemisiifolia replacement during natural or "artificial" succession. Acta Oecol. 82, 32–forty. doi: ten.1016/j.actao.2017.05.007
CrossRef Full Text | Google Scholar
Ghiani, A., Aina, R., Asero, R., Bellotto, E., and Citterio, S. (2012). Ragweed pollen nerveless along high-traffic roads shows a college allergenicity than pollen sampled in vegetated areas. Allergy 67, 887–894. doi: 10.1111/j.1398-9995.2012.02846.ten
PubMed Abstract | CrossRef Full Text | Google Scholar
Ghiani, A., Ciappetta, S., Gentili, R., Asero, R., and Citterio, S. (2016). Is ragweed pollen allergenicity governed past environmental weather condition during constitute growth and flowering? Sci. Rep. 6:30438. doi: x.1038/srep30438
PubMed Abstruse | CrossRef Full Text | Google Scholar
Gilbert, B., and Lechowicz, Chiliad. J. (2005). Invasibility and abiotic gradients: the positive correlation between native and exotic constitute multifariousness. Ecology 86, 1848–1855. doi: 10.1890/04-09997
CrossRef Full Text | Google Scholar
Goto, Y., Kondo, T., Hayashi, Eastward., Kuramoto, North., Takahashi, M., and Yasueda, H. (2004). Influences of genetic and environmental factors on the concentration of the allergen Cry j i in sugi (Cryptomeria japonica) pollen. Tree Physiol. 24, 409–414. doi: ten.1093/treephys/24.four.409
PubMed Abstruse | CrossRef Full Text | Google Scholar
Hao, J. H., Lv, S. S., Bhattacharya, S., and Fu, J. G. (2017). Germination response of iv conflicting congeneric Amaranthus species to environmental factors. PLoS ONE 12:e0170297. doi: x.1371/periodical.pone.0170297
PubMed Abstract | CrossRef Full Text | Google Scholar
Hinsinger, P., Plassard, C., Tang, C., and Jaillard, B. (2003). Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248, 43–59. doi: 10.1023/A:1022371130939
CrossRef Full Text | Google Scholar
Islam, A. K. M. S., Edwards, D. G., and Asher, C. J. (1980). pH optima for crop growth. Plant Soil 54, 339–357. doi: x.1007/BF02181830
CrossRef Full Text | Google Scholar
Jensen, T. Fifty. (2010). Soil pH and the Availability of Plant Nutrients, International Found Diet Institute (IPNI). Plant Diet TODAY.
Jiang, Y., Li, Y., Zeng, Q., Wei, J., and Yu, H. (2017). The result of soil pH on plant growth, leafage chlorophyll fluorescence and mineral chemical element content of two blueberries. Acta Hort. 1180, 269–276. doi: 10.17660/ActaHortic.2017.1180.36
CrossRef Total Text | Google Scholar
Kleinbaum, D. Thousand., and Klein, Thou. (2005). Survival Assay. A Self-Learning Text, 2nd Edn. New York, NY: Springer, 596.
Google Scholar
Köpp, M. Chiliad., Passos, L. P., da Silva Verneue, R., da Silva Lédo, F. J., Meirelles Coimbra, J. L., and Costa de Oliveira, A. (2011). Effects of nutrient solution pH on growth parameters of alfalfa (Medicago sativa L.) genotypes. Commun. Sci. 2, 135–141.
Google Scholar
Kulmatiski, A., and Beard, K. H. (2006). Activated carbon as a restoration tool: potential for control of invasive plants in abandoned agronomical fields. Restor. Ecol. 14, 251–257. doi: 10.1111/j.1526-100X.2006.00127.10
CrossRef Full Text | Google Scholar
Lankinen, Å. (2000). Effects of soil pH and phosphorus on in vitro pollen competitive power and sporophytic traits in clones of Viola tricolor. Int. J. Plant Sci. 161, 885–893. doi: x.1086/317561
CrossRef Full Text | Google Scholar
Leskovsek, R., Datta, A., Simoncic, A., and Knezevic, Due south. Z. (2012). Influence of nitrogen and plant density on the growth and seed production of common ragweed (Ambrosia artemisiifolia L.). J. Pest. Sci. 85,527–539. doi: 10.1007/s10340-012-0433-ii
CrossRef Full Text
Li, H. Due north., Xiao, B., Liu, W. X., and Huan, F. H. (2014). Changes in Soil Biota Resulting from Growth of the Invasive Weed, Ambrosia artemisiifolia 50. (Aster family), Enhance Its Success and Reduce. J. Integr. Agr. thirteen, 1962–1971. doi: 10.1016/S2095-3119(13)60569-9
CrossRef Total Text | Google Scholar
Lommen, S. T. Eastward., Ciappetta, Due south., Ghiani, A., Asero, R., Gentili, R., Müller-Schärer, H., et al. (2017). Defoliation of common ragweed by Ophraella communa beetle does non bear on pollen allergenicity in controlled conditions. Plant Biosyst. 151, 1094–1100. doi: ten.1080/11263504.2016.1244122
CrossRef Total Text | Google Scholar
Lončarić, Z., Karalić, M., Popović, B., Rastija, D., and Vukobratović, K. (2008). Total and plant bachelor micronutrients in acidic and calcareous soils in Croatia. Cereal Res. Commun. 36, 331–334. doi: 10.1556/CRC.36
CrossRef Total Text | Google Scholar
Mannino, A. M., and Balistreri, P. (2018). Denizen Science: A Successful Tool for Monitoring Invasive Alien Species (IAS) in Marine Protected Areas. The case written report of the Egadi Islands MPA, Tyrrhenian Bounding main, Italian republic, Biodiversity.
Mohebbi, South., and Mahler, R. L. (1989). The effect of soil pH on wheat and lentils grown on an agriculturally acidified northern Idaho soil under greenhouse conditions. Commun. in Soil Sci. Plant Anal. 20, 359–381. doi: 10.1080/00103628909368088
CrossRef Total Text | Google Scholar
Montagnani, C., Gentili, R., Smith, M., Guarino, M. F., and Citterio, S. (2017). The worldwide spread, success, and impact of ragweed (Ambrosia spp.). Crit. Rev. Establish Sci. 36, 139–178. doi: x.1080/07352689.2017.1360112
CrossRef Total Text | Google Scholar
Morganti, Thousand., Rubolini, D., Akesson, Southward., Bermejo, A., De la Puente, J., Lardelli, R., et al. (2017). Effect of lite-level geolocators on apparent survival of two highly aeriform swift species. J. Avian Biol. 49, doi: 10.1111/jav.01521
CrossRef Full Text | Google Scholar
Nádasi, Eastward., and Kazinczi, One thousand. (2011). Growth of common ragweed (Ambrosia artemisiifolia) on different soil types with various nitrogen supplies in Proceedings of the tenth Slovenian Conference on Establish Protection (Podčetrtek, Solvenia), one–2.
Google Scholar
Offord, C. A., Meagher, P. F., and Zimmer, H. C. (2014). Growing upwardly or growing out? How soil pH and light affect seedling growth of a relictual rainforest tree. AoB Plants 6:plu011. doi: 10.1093/aobpla/plu011
PubMed Abstract | CrossRef Full Text | Google Scholar
Oswald, S. A., Nisbet, I. C. T., Chiaradia, A., and Arnold, J. M. (2012). FlexParamCurve: R package for flexible fitting of nonlinear parametric curves. Methods Ecol. Evol. iii, 1073–1077. doi: 10.1111/j.2041-210X.2012.00231.x
CrossRef Full Text | Google Scholar
Paine, C. E. T., Marthew, T. R., Vogt, D. R., Purves, D., Rees, M., Hector, A., et al. (2012). How to fit nonlinear institute growth models and summate growth rates: an update for ecologists. Methods Ecol. Evol. 3, 245–256. doi: ten.1111/j.2041-210X.2011.00155.x
CrossRef Total Text | Google Scholar
Pansu, M., and Gautheyrou, J. (2003). Fifty'analyse du sol, Minéralogique et Minérale. Berlin; Heidelberg New: Springer-Verlag.
Google Scholar
Patracchini, C., Vidotto, F., and Ferrero, A. (2011). Common ragweed (Ambrosia artemisiifolia) Growth equally affected by plant density and clipping. Weed Technol. 25, 268–276. doi: ten.1614/WT-D-09-00070.ane
CrossRef Full Text | Google Scholar
Pignatti, S., Menegoni, P., and Pietrosanti, Southward. (2005). Biondicazione attraverso le piante vascolari. Valori di indicazione secondo Ellenberg (Zeigerwerte) per le specie della Flora d'Italia. Braun-Blanquetia 39, ane–97.
Google Scholar
Pinheiro, J. C., and Bates, D. M. (1995). Approximation to the log-likelihood function in the nonlinear mixed-effects model. J. Comput. Graph. Statist. 4, 12–35.
Google Scholar
Pinke, Thou., Karácsony, P., Czúcz, B., and Botta-Dukát, Z. (2011). Ecology and land-use variables determining the abundance of Ambrosia artemisiifolia in arable fields in Hungary. Preslia 83, 219–235.
Google Scholar
Pinke, G., Karácsony, P., Czúcz, B., Botta-Dukát, Z., and Lengyel, A. (2012). The influence of environment,management and site context on species composition of summer arable weed vegetation in Hungary. Appl. Veg. Sci. 15, 136–144. doi: 10.1111/j.1654-109X.2011.01158.x
CrossRef Total Text | Google Scholar
Qin, Z., Xie, J. F., Quan, G. K., Zhang, J. N., Mao, D. J., and DiTommaso, A. (2014). Impacts of the invasive annual herb Ambrosia artemisiifolia L. on soil microbial carbon source utilization and enzymatic activities. Europ. J. Soil Biol. 60, 58–66. doi: 10.1016/j.ejsobi.2013.xi.007
CrossRef Total Text | Google Scholar
R Cadre Team (2016). R: A Language and Surround for Statistical Calculating. Vienna: R Foundation for Statistical Computing. Bachelor online at: https://www.R-projection.org/
Ramírez-Rodríguez, Five., López-Bucio, J., and Herrera-Estrella, 50. (2005). Adaptive response in plants to nonoptimal soil pH in Found Abiotic Stress, eds M. A. Jenks and P. Grand. Hasegawa (Oxford: Blakwell Publishing), 145–150.
Google Scholar
Richardson, D. M., and van Wilgen, B. (2004). Invasive alien plants in South Africa: how well do nosotros sympathize the ecological impacts? South. Afr. J. Sci. 100, 45–51.
Google Scholar
Robson, A. D. (1989). Soil Acidity and Plant Growth. Sydney,NSW: Academic Printing.
Google Scholar
Rosta, H. R., and Rezaei, H. (2014). Consequence of nutrient solution PH on the vegetative and reproductive growth and physiological characteristics of Rose Cv. 'Grand Gala' in hydroponic system. J. Plant Nutr. 37, 2179–2194. doi: 10.1080/01904167.2014.920377
CrossRef Full Text | Google Scholar
Sang, W., Liu, Ten., and Axmacher, J. C. (2011). Germination and emergence of Ambrosia artemisiifolia L. under changing ecology atmospheric condition in Communist china. Plant Spec. Biol. 26, 125–133. doi: 10.1111/j.1442-1984.2011.00314.x
CrossRef Total Text | Google Scholar
Sǎrǎteanu, Five., Moisuc, A., and Cotuna, O. (2010). Ambrosia artemisiifolia L. an invasive weed from ruderal areas to disturbed grasslands. Lucrǎri Stiinifice 53, 28–31.
Google Scholar
Serna, G., Borras, R., Legaz, F., and Primo-Millo, E. (1992). The influence of nitrogen concentration and ammonium/nitrate ratio on n-uptake, mineral composition and yield of citrus. Plant Soil 147, 13–23. doi: x.1007/BF00009366
CrossRef Full Text | Google Scholar
Sicurella, B., Caprioli, M., Romano, A., Romano, M., Rubolini, D., Saino, Northward., et al. (2014). Hayfields raise colony size of the barn consume Hirundo rustica in northern Italy. Bird Conserv. Int. 24, 17–31. doi: 10.1017/S095927091300021X
CrossRef Total Text | Google Scholar
Skálová, H., Moravcová, L., Dixon, A. F. G., Kindlmann, P., and Pyšek, P. (2015). Effect of temperature and nutrients on the growth and development of seedlings of an invasive plant. AoB Plants 7:plv044. doi: ten.1093/aobpla/plv044
PubMed Abstruse | CrossRef Full Text | Google Scholar
Smith, M., Cecchi, Fifty., Skjøth, C. A., Karrer, G., and Šikoparija, B. (2013). Common ragweed: a threat to ecology wellness in Europe. Environ. Int. 61, 115–126. doi: ten.1016/j.envint.2013.08.005
PubMed Abstract | CrossRef Total Text | Google Scholar
Lord's day, S., and Frelich, L. E. (2011). Flowering phenology and height growth pattern are associated with maximum plant top, relative growth rate and stalk tissue mass density in herbaceous grassland species. J. Ecol. 99, 991–1000. doi: ten.1111/j.1365-2745.2011.01830.10
CrossRef Full Text | Google Scholar
Szigetvári, C., and Benkö, Z. R. (2008). Mutual ragweed (Ambrosia elatior L.) in The Almost Important Invasive Plants in Hungary, eds Z. Botta-Dukát and L. Balogh (Vácrátót, Hungary: Hungarian Academy of Sciences, Establish of Environmental and Botany), 189–201.
Google Scholar
Tessmer, O. L., Jiao, Y., Cruz, J. A., Kramer, D. M., and Chen, J. (2013). Functional approach to high-throughput plant growth analysis. BMC Syst. Biol. 7:S17. doi: ten.1186/1752-0509-7-S6-S17
PubMed Abstract | CrossRef Full Text | Google Scholar
Therneau, T. Thousand., and Grambsch, P. M. (2000). Modeling Survival Information: Extending the Cox Model. New York, NY: Springer; Verlag.
Google Scholar
Thompson, W. H., McFarland, C., and Brown, T. (2016). Agricultural Lime and Liming Part 2: Laboratory Testing to Decide Lime Requirements. Washington, DC: Washington State University Fact Sail.
Google Scholar
Turner, J. A. (1928). Relation of the distribution of certain Compositae to the hydrogen-ion concentration of the soil. Bull. Torrey Bot. Club 55, 199–213. doi: 10.2307/2480472
CrossRef Full Text | Google Scholar
Ujvárosi, M. (1973). Gyomnövények [Weeds]. Budapest: Mezogazdasági Kiadó.
Vasquez, E., Sheley, R., and Svejcar, T. (2008). creating invasion resistant soils via nitrogen management. Invas. Plant Sci. Mana. 1, 304–314. doi: 10.1614/IPSM-07-059.1
CrossRef Full Text | Google Scholar
Vilá, Thou., Espinar, J. L., Hejda, M., Hulme, P. E., Jarošík, Five., Maron, J. 50., et al. (2011). Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 14, 702–708. doi: 10.1111/j.1461-0248.2011.01628.ten
PubMed Abstract | CrossRef Full Text | Google Scholar
Ware, S. (1990). Adaptation to substrate—And lack of it—In rock outcrop plants: Sedum and Arenaria. Amer. J. Bot. 77, 1095–1100. doi: 10.1002/j.1537-2197.1990.tb13605.10
CrossRef Full Text | Google Scholar
Yin, X., Goudriaan, J., Lantinga, E. A., Vos, J., and Spiertz, H. J. (2003). A flexible sigmoid office of determinate growth. Ann. Bot. 91, 361–371. doi: ten.1093/aob/mcg029
PubMed Abstract | CrossRef Full Text | Google Scholar
Zeng, Y., and Clark, Thou. J. (2013). Optimal growing substrate pH for v Sedum species. HortScience 48, 448–452.
Google Scholar
Zollinger, R. Thousand., and Kells, J. J. (1991). Effects of soil pH, soil water, low-cal intensity, and temperature on perennial sowthistle (Sonchus arvensis 50.). Weed Sci. 39, 376–384.
Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fpls.2018.01335/full
0 Response to "Which Ph Reading Is Suitable for Most Plants and Soil Microorganisms?"
Post a Comment